Our Technology
At Helius, we pride ourselves on being at the forefront of developing technology that is essential in addressing the needs of neurological disorders that effect movement and coordination.

The Science
The Scientific Theory: Improve Impaired Function by Stimulating Cranial Nerves via the Tongue
Our technology is based on almost 60 years of research in the field of neuromodulation—the use of external stimulation to regulate the electrochemical environment of the brain.
In clinical studies, the PoNS device coupled with targeted functional therapy (together PoNS Therapy™) induces neuroplasticity. PoNS Therapy consists of balance and gait training and breathing and awareness exercises, as well as other neurorehabilitation exercises.
The Brainchild
This novel way to apply the concept of neuroplasticity to rehabilitation is the brainchild of scientists at the University of Wisconsin-Madison Tactile Communication and Neurorehabilitation Laboratory (TCNL).
Studying Correlation of Sensory Motor Cranial Nerves and Function
It is believed that neuromodulation triggers neuroplasticity, the brain’s ability to restructure or relearn in response to new experiences, sensory input, and functional demands.
Understanding the Role of Neuroplasticity in Functional Rehabilitation
Through extensive research, the role of neuroplasticity in functional rehabilitation for gait and balance is now better understood. Research results have shown that the process of neuroplasticity underlies all cerebral learning, training, and rehabilitation.
Physical therapy exercise may trigger neurostimulation mechanisms that deliver signals to specific neurological sites in the body.
This process, known as therapeutic neuromodulation, leads to alteration of nerve activity that can help modulate abnormal neural pathways’ behavior caused by the disease process and re-establishing neural balance.
Neuromodulation can promote changes in neural activity and induce mechanisms of neural network repair, called adaptive changes, which can compensate for the disrupted functional processes, which is the effect of neuroplasticity.
The PoNS® Device
The PoNS Device and how trigeminal translingual delivery of therapy activates mechanisms that result in neuroplasticity.
Why the tongue?
The tongue, which has a large cortical representation, is innervated in the front two-thirds by branches of the trigeminal and facial nerves with direct connections to the brainstem.

The trigeminal nerve (lingual)
Responsible for sensations in the face, biting and chewing
The facial nerve (chorda tympani)
Responsible for motor control of most of the muscles of facial expression
The electrical stimulation of the cranial nerves creates a flow of neural impulses that are then delivered directly into the brain stem—the main control center for many life functions, including sensory perception, movement, and balance.
Clinical evidence suggests that PoNS Therapy™ (PoNS device plus physical therapy) can exert neuromodulation effects by the translingual stimulation of the trigeminal and facial nerve branches.
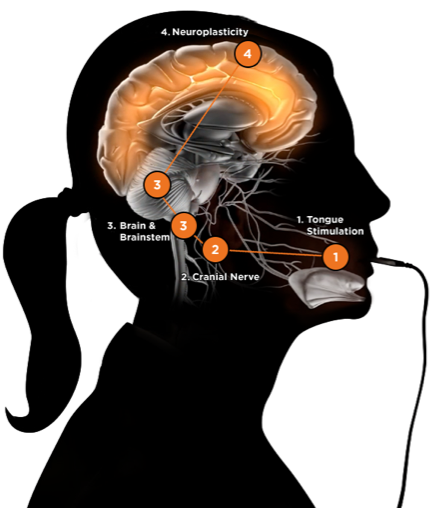
Brainstem Activity Modulation
Brainstem activity leads to upregulation of cortical activity that mediates function. The 2 cranial nerves (trigeminal and facial) have direct connections with specific areas of the brainstem (in the pons varolii and the medulla) and, consequently, promote activation of target regions in the cerebral cortex, including the left motor cortex, the bilateral anterior cingulate, and the dorsolateral prefrontal cortex areas.
Triggering Neuroplastic Changes in the Brain Over Time
With sustained neuromodulation, the brain, through neuroplasticity, may consolidate these adaptive changes and “learn” to employ the new compensatory mechanisms and pathways to improve or regain control of the impaired function.
Continuous translingual neuromodulation, over 14 weeks of physical therapy, is likely to induce neuroplastic changes that help replace impaired corticospinal pathways and deliver the signal to the spinal cord. This neuroplastic process may ultimately be responsible for improved function and consolidated therapeutic effects over the treatment period.
Clinical Trials
PoNS Therapy Clinical Studies Across the Globe
Over the last few decades, there have been exciting advances in the field of neuroplasticity, which is the ability of the brain to form and reorganize synaptic connections, especially in response to learning or experience, or after injury. Helius has studied the PoNS® to determine whether its use in combination with physical therapy can improve balance and gait deficit as a result of a mild-to-moderate traumatic brain injury (mmTBI), and mild to moderate multiple sclerosis (MS).
PoNS Therapy™ has been independently studied in other neurological diseases, including subacute stroke.1
PoNS has received breakthrough designation for treatment of multiple sclerosis and stroke
In 2020, Helius received FDA breakthrough designation for PoNS treatment of gait deficit due to symptoms of MS and submitted request for FDA de novo classification.
In 2021, Helius received FDA breakthrough designation for PoNS treatment of gait deficit due to stroke.
PoNS® Authorization for Use
The Portable Neuromodulation Stimulator (PoNS®) is an authorized medical device commercially available in the US for the treatment of gait deficit due to Multiple Sclerosis (MS) and in Canada for the treatment of balance deficit due to mild to moderate Traumatic Brain Injury, gait deficit due to Multiple Sclerosis (MS) and gait deficit due to stroke.
PoNS® is authorized in Australia (AUS TGA) for use as an adjunct to a therapeutic exercise program to improve balance and gait. It is not yet commercially available.
For more information on PoNS in the US, visit ponstherapy.com.
For more information on PoNS in Canada, visit ponstherapy.ca.
REFERENCE: 1. Galea MP, Cofré Lizama LE, Bastani A, Panisset MG, Khan F. Cranial nerve non-invasive neuromodulation improves gait and balance in stroke survivors: a pilot randomised controlled trial. Brain Stimul. 2017;10(6):1133-1135. doi: 10.1016/j.brs.2017.08.011
Helius News
Careers at Helius Medical
At Helius, you can have a positive impact on the lives of millions of people around the globe who rely on our innovations for their recovery. Helius brings together the world’s best talent to succeed in our ongoing mission. Together, we work passionately to develop and deliver easy-to-use technology for neurological wellness. If you want to join us in our mission, we want to hear from you.
